




Acne is a chronic inflammatory disease affecting the pilosebaceous follicle, characterized by different type of lesions. Three main factors are implicated in the development of acne lesions:
Inflammation is crucial in the development of acne and could potentially contribute to the development of scars. It has been located in active lesions and is also at the origin of persistent post-inflammatory hyperpigmentation by stimulation of alpha melanocyte-stimulating hormone and interleukin (IL)-1.
Skin is colonized by a dense community of commensal microorganisms including bacteria, viruses and fungi. Changes in the composition of cutaneous microbial communities have been linked to several chronic inflammatory skin diseases, including atopic dermatitis, psoriasis and acne. Cutaneous bacterial communities are involved in immune homeostasis1.
Improved understanding about interactions between skin microbiome and the innate immune response in acne should help physicians to design efficacious treatment strategies, specifically concerning the role of dermocosmetics on the skin microbiome.
The skin microbiota is strongly influenced by numerous conditions. Sex and age of a person, genetics, diet, environmental factors (both climatic and geographic), immune response, lifestyle (e.g. occupation or hygiene) and underlying disease play a critical role in driving and regulating the composition of our skin microbiota2.
Considering the skin, microbiota is present on the surface as well as deep within the dermal compartment owing to connections with structures such as the sebaceous glands3. Interestingly, microorganisms has been found to be in close dialogue with other cell types, including resident immunologically important cells (e.g. T-cells). In addition, it has been shown that microorganisms in contact with Langerhans cells bridge the innate and the adaptive immune system, allowing local production of antimicrobial peptides (AMPs)4. Although diverse colonization is important for our health as our microbiota consistently delivers signals that contribute to the education of our immune system5.
C. acnes is a commensal bacteria of the pilosebaceous follicle. This anaerobic bacterium grows particularly in the sebaceous areas of the forehead, retro-auricular crease and back2. It’s playing a physiological role by inhibiting the invasion of pathogenic bacteria such as S. aureus and S. pyogenes. In addition, C. acnes maintain the acidic pH in the skin including sebaceous gland by hydrolyzing triglycerides, releasing free fatty acids and secreting propionic acid2.
C. acnes interacts with the innate immune system to promote inflammation in two different ways.
Recent studies have shown that acne is not necessarily only the result of the proliferation of C. acnes9. Genome comparison of C. acnes strains allowed identification of different profiles of commensal C. acnes subtypes between healthy skin and acne lesions.
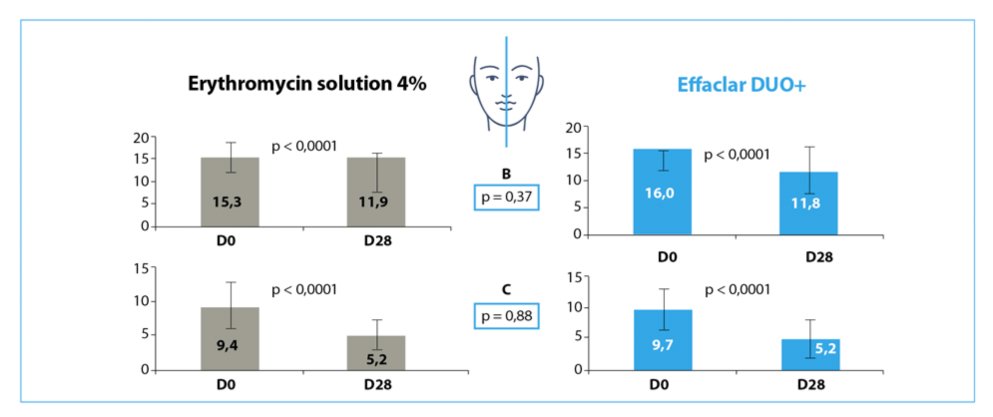
Reduction of the number of B=Papulo-pustule and C= comedone in both hemi-face after 28 days of treatment with either erythromycin 4% or Effaclar Duo+ (n=26, mean±SD).
In collaboration with L’Oréal Research and Innovation and the University of Boulder (Colorado, USA), a study has been initiated with the Pr B. Dreno (Nantes, France), with 2 objectives:
This single-center, controlled, randomized, double blinded, intraindividual (split-face) comparison was conducted in 55 acne patients grade 2, 3 (GEA Grading) before and after a 28 days treatment:
After 28 days treatment a significant reduction of both comedonal and inflammatory lesions was reported with no significant difference between the dermocosmetic and erythromycin 4% (Figure).
Superficial skin microbiota was characterized by swabbing, taken under axenic conditions; from 3 adjacent sites (come done, papulo-pustule and clinically normal skin). A high-throughput sequencing approach was used: it targets a portion of the 16S rRNA bacterial gene. 16S rRNA is specific to each bacterium and allows the identification of the bacterial diversity and landscape at the skin surface of acne patients9.
Results indicate a low bacterial biodiversity, varying with the location of the sampled areas (e.g. cheek versus forehead) Nevertheless, the bacterial diversity is similar on all the 3 sampled areas and do not vary with the severity of can (grade2 versus grade3) (p=0.39).
Shannon index (n=26 - mean±SD):
Clinically normal skin = 2, 82 ± 0, 90
Papulo-pustule = 2, 65 ± 0, 89
Comedone = 2, 90 ± 0, 54
Interestingly, 4% erythromycin acted mainly on actinobacteria (including Corynebacterium and Propionibacterium). The dermocosmetic acted on actinobacteria and also on Staphylococcus.
These data demonstrate that in acneic patients’ normal skin, comedone and papulo-pustule have similar low superficial bacterial diversity. The most important information is that Staphylococcus is the predominant bacterial genus both on superficial skin of acne lesions and normal looking skin, before and after both treatments, and not Propionibacterium.
In conclusion, Effaclar Duo+ in monotherapy, in adjunctive therapy or after therapeutic treatments (which target mainly Propionibacterium) offers a good strategy to manage overabundance of Staphylococcus at the skin surface of acne-affected patients
This article has been written by S. Seite at Laboratoires dermatologiques La Roche-Posay (Asnières, France)
Bibliography